Evonik invests US$220 million in partnership with the U.S. Government to build new lipid production facility for mRNA-based therapies in the U.S.
- Important contribution to the development of new mRNA therapies beyond COVID-19 vaccines
- Tippecanoe site in Lafayette, Indiana, will enable flexible production of lipids
- The Biomedical Advanced Research and Development Authority (BARDA), within the Office of the Assistant Secretary for Preparedness and Response (ASPR) in the U.S. Department of Health and Human Services provides funding to strengthen nation’s vaccine preparedness
- Indiana Economic Development Corporation, Greater Lafayette Commerce, and Duke Energy support the project
- Investment to create more than 80 highly skilled jobs in the Lafayette region
Essen, Germany/Parsippany, USA. Evonik, one of the world's leading providers of drug delivery technologies, is building a new, highly flexible, global-scale production facility for pharmaceutical lipids in the United States. The new plant at Evonik’s Tippecanoe site in Lafayette, Indiana, will broadly position the Group for future growth in novel mRNA-based therapies beyond COVID-19 vaccines and strengthen its leading role as a strategic partner for innovative pharmaceutical companies worldwide. Construction will begin in early 2023, and the plant is scheduled to go onstream in 2025. The investment into the lipid facility will help create more than 80 highly skilled jobs in the Lafayette region.
The total investment amounts to US$220 million. The U.S. Government is funding the facility with up to US$150 million through its Biomedical Advanced Research and Development Authority (BARDA), a component of the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health and Human Services. BARDA promotes the advanced development of medical countermeasures to respond to 21st century health security threats and coordinated contracting support with the support of the DOD Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND). Additional support will be provided by the Indiana Economic Development Corporation (IEDC), Greater Lafayette Commerce (GLC), and Duke Energy.
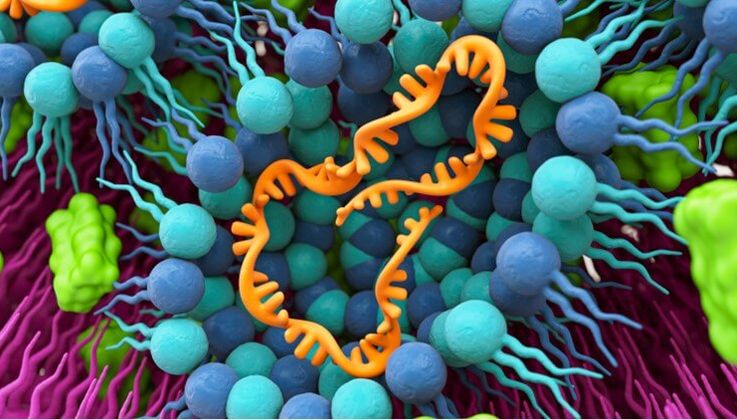
The Health Care business of Evonik, a specialty chemicals company headquartered in Essen, Germany, serves as a leading and integrated service provider of products and technologies for mRNA-based medicines. Evonik has been supplying major pharmaceutical companies worldwide with the lipids needed for use with mRNA active ingredients (messenger ribonucleic acid). Lipids are critical components in the formulation of nucleic acid therapies.
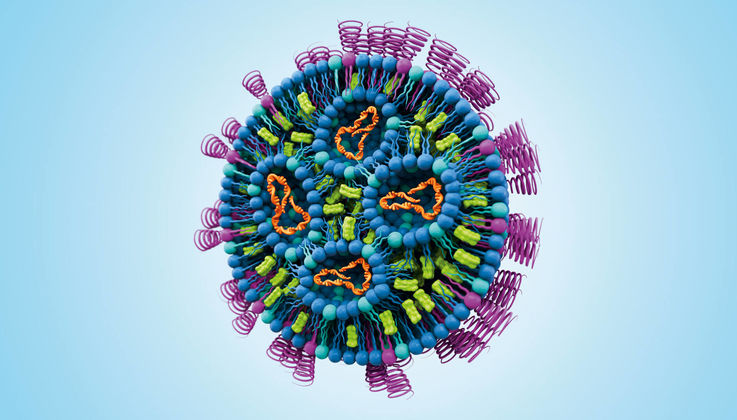
During the coronavirus pandemic, Evonik made a crucial contribution by providing lipids to the Pfizer/BioNTech COVID-19 vaccine and vaccination campaigns worldwide. mRNA serves as a carrier of genetic information in cells. It can be designed for a broad range of pharmacological applications. mRNA vaccines, for example, teach cells how to make a protein that will trigger an immune response.
“With this investment in lipid production, we are further expanding our leading position on the global market and specifically strengthening our Health Care business,” says Evonik CEO Christian Kullmann. “It supports our strategic transformation into ‘Next Generation Evonik’, contributing value-added solutions with superior environmental and socio-economic profiles to our customers.”
The Tippecanoe site in Indiana is Evonik’s preferred location for this project due to its existing infrastructure, a highly skilled workforce, and readily available technologies. Tippecanoe is one of the world’s largest sites for active pharmaceutical ingredients (APIs) and Evonik's second-largest site in the U.S., with around 650 employees. Evonik is a leading producer of APIs, focusing on large-scale manufacturing of highly potent drug substances and APIs based on complex chemistry which require multi-step synthesis.
“The U.S. Government is pleased to be a part of these Industrial Base Expansion efforts to expand production of raw materials for mRNA vaccines,” says Nicole Kilgore, Deputy Joint Program Executive Officer (JPEO) for Chemical, Biological, Radiological and Nuclear (CBRN) Defense.
“Evonik’s strategic expansion at Tippecanoe is fantastic news for the entire Greater Lafayette region,” said Scott Walker, president and CEO of Greater Lafayette Commerce. “The Town of Shadeland, Lafayette, West Lafayette, Tippecanoe County, the Purdue Research Foundation, and Greater Lafayette Commerce all supported this effort and not only look forward to the high-value jobs it will create, but also the important role the new Lipid Center of Excellence will play in our nation’s vaccine preparedness.”
By expanding the production of specialty lipids, Evonik is strengthening the Nutrition & Care division's portfolio of system solutions for advanced drug delivery. The division aims to increase the share of system solutions from 20 percent today to more than 50 percent by 2030.
“As a strategic partner for pharma and biotech companies, we are using the new facility to support our customers in developing nucleic acid-based drugs right up to commercialization. These new therapies are the future,” says Thomas Riermeier, head of Evonik's Health Care business. “We are also evaluating further expansion of our formulation services and scale-up capacity, thereby consolidating our leading position as an end-to-end provider.” Pharmaceutical formulation is a multi-step process of mixing the active drug with all other components.
The new multi-purpose facility will allow the rapid and flexible production of a variety of lipids. These will serve future applications of mRNA technology in infectious disease control, cancer immunotherapy, protein replacement, and gene therapy. Furthermore, the new facility ensures a rapid and extensive supply of lipids as needed in case of a future pandemic.
Lipids, molecules that make up the building blocks of living cells, are critical to producing mRNA-based drugs. The mRNA is enclosed in a lipid nanoparticle (LNP) composed of specific lipids. The LNP protects the mRNA and delivers it safely into the cell, where it is released. LNPs are currently the most advanced drug delivery system and have gained worldwide acceptance in the fight against COVID-19 due to their versatility.
Evonik recognized the potential of gene-based therapeutic approaches early on and made targeted investments in this technology back in 2016 with the acquisition of the Canadian company Transferra Nanosciences. Evonik’s laboratories in Vancouver focus on developing lipid-based, parenteral drug formulations, including LNPs and liposomes. Evonik expanded its portfolio in 2020 with the acquisition of Wilshire Technologies, an American manufacturer of plant-based excipients for the pharmaceutical industry. Excipients are non-pharmaceutically active ingredients and, as in case of lipids, can play a crucial role to help the APIs to reach the designation in the body.
As a partner to the pharmaceutical industry, Evonik has been a leader in advanced drug delivery for decades. It supports pharmaceutical companies worldwide with comprehensive services for developing and manufacturing complex parenteral and oral drug products.
For more information please visit:
Company information
Evonik is one of the world leaders in specialty chemicals. The company is active in more than 100 countries around the world and generated sales of €15 billion and an operating profit (adjusted EBITDA) of €2.38 billion in 2021. Evonik goes far beyond chemistry to create innovative, profitable and sustainable solutions for customers. About 33,000 employees work together for a common purpose: We want to improve life today and tomorrow.
About Nutrition & Care
The focus of the business of the Nutrition & Care division is on health and quality of life. It develops differentiated solutions for active pharmaceutical ingredients, medical devices, nutrition for humans and animals, personal care, cosmetics, and household cleaning. In these resilient end markets, the division generated sales of €3.56 billion in 2021 with about 5,300 employees.
Disclaimer
- In so far as forecasts or expectations are expressed in this press release or where our statements concern the future, these forecasts, expectations, or statements may involve known or unknown risks and uncertainties. Actual results or developments may vary, depending on changes in the operating environment. Neither Evonik Industries AG nor its group companies assume an obligation to update the forecasts, expectations, or statements contained in this release.
- The Army Contracting Command – Aberdeen Proving Ground – COVID Response is the awarding and administering acquisition office. This work was supported by BARDA and the JPEO-CBRND, through the Industrial Base Expansion program under Award Number IBx W58P05-22-2-0006. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the U.S. government.
- This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under Contract No. W58P05-22-2-0006.